Currently, there are locally confirmed cases or asymptomatic infections in Shaanxi, Henan, Tianjin, Beijing, Shanghai, Guangdong and many other places, and the epidemic shows a state of multi-point occurrence and local outbreak nationwide. In order to guide the work of the pooled sample testing for the SARS-CoV-2 around and to further improve the ability and efficiency of the nucleic acid test, on January 15, the Joint Prevention and Control Mechanism of the State Council for COVID-19 (Medical Treatment Team), based on the existing 10 pooled samples in one tube for SARS-CoV-2 nucleic acid test, published the Technical Specification for 20 Pooled Samples in One Tube for SARS-CoV-2 Nucleic Acid Test, which puts higher requirements on the corresponding supporting sampling tube consumables, the sensitivity of nucleic acid testing reagents and the performance of the supporting instruments and devices.
Three-target High-sensitivity Testing Protocol
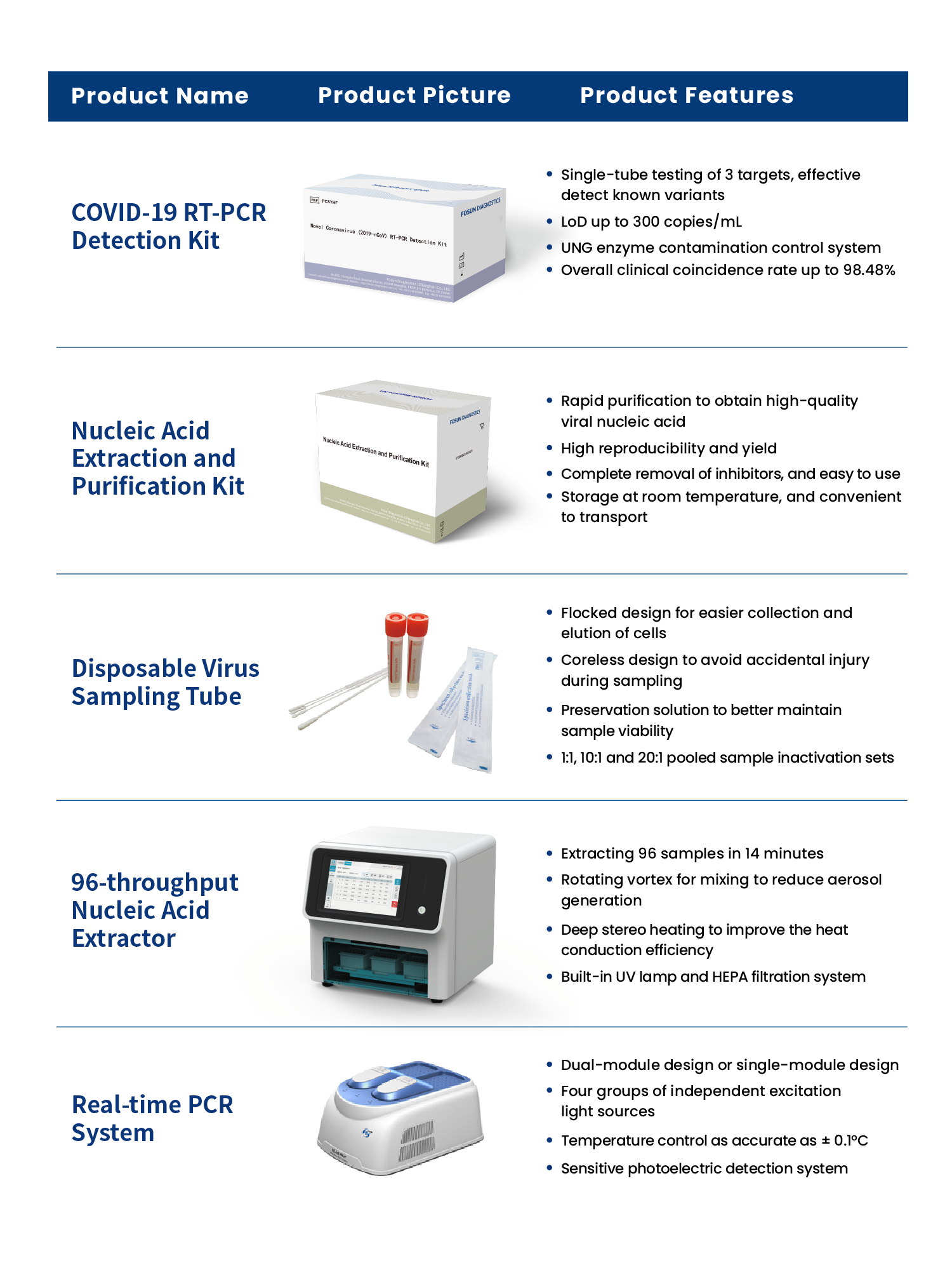
In the face of the need for large-scale screening in a short period of time, the pooled sample testing for SARS-CoV-2 can be used for primary screening to improve testing capacity and efficiency, and the choice of using a highly sensitive and validated nucleic acid testing protocol is of great importance to reduce missed cases. Fosun Diagnostics can provide a complete solution including reagents, equipment, and consumables for the SARS-CoV-2 nucleic acid test, which has been comprehensively validated for its performance and has stronger advantages in terms of rapid speed and safety, flexible throughput, high sensitivity and reliable results.
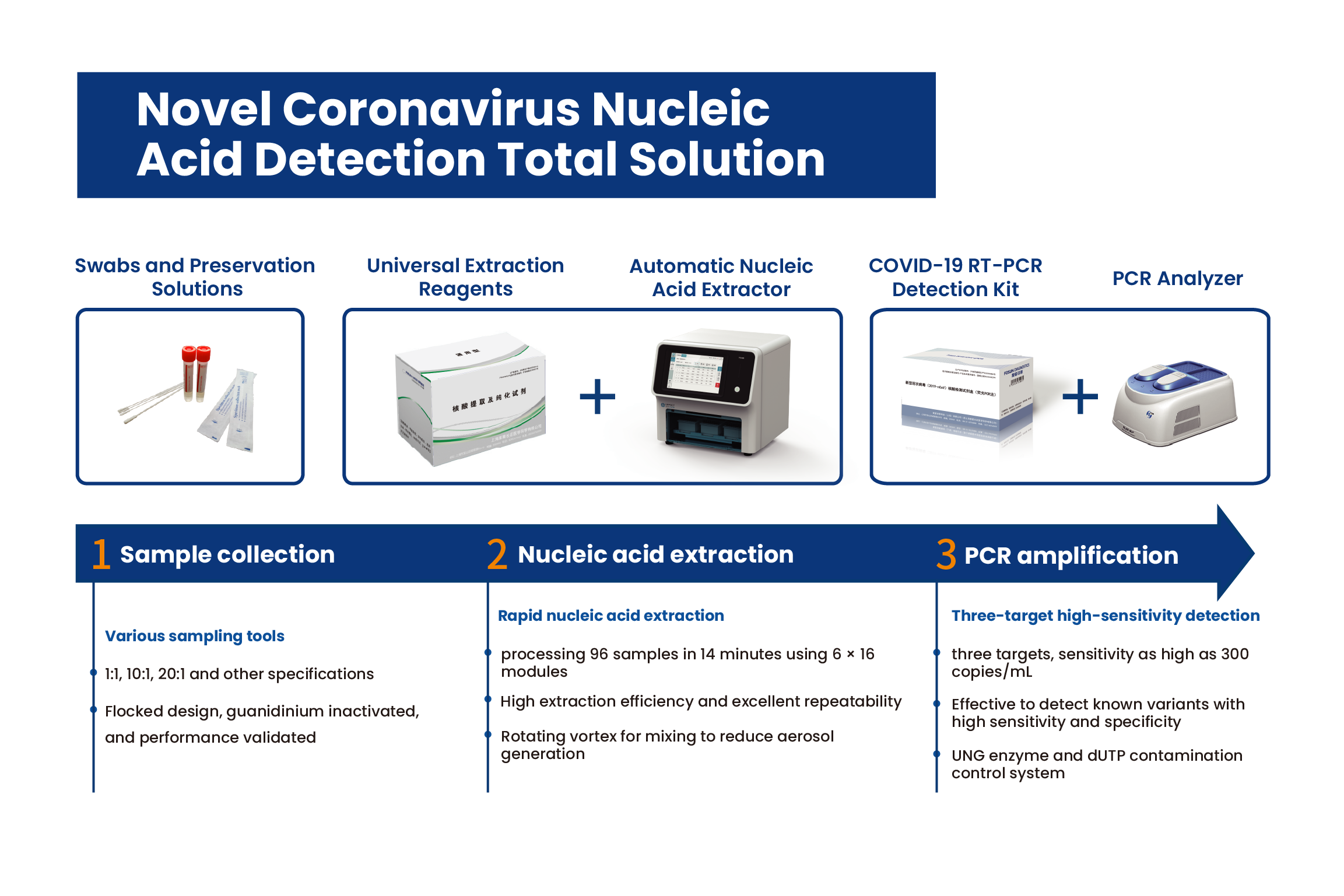
Product Advantages
High sensitivity is essential for pooled sample testing
In large-scale population screening, as the prevalence among the population is very low (<0.1%), it is particularly important to use a more sensitive test method for the infected population with relatively low levels of virus or asymptomatic population to avoid missed cases. If the test method is not sensitive enough, a negative result only means that someone is less likely to be infected, not that he/she is not carrying the virus. A false negative result will result in very serious consequences, as the infected or asymptomatic population may infect others for not being quarantined.
On December 28, 2020, the Joint Prevention and Control Mechanism of the State Council issued the Workbook for SARS-CoV-2 Nucleic Acid Test in Medical Institutions (Trial Edition 2), which requires strengthening the quality control of nucleic acid tests and recommends using highly sensitive reagents (LOD≤ 500 copies/mL) and reagents with more than two target segments for testing to reduce the risk of possible missed cases due to insufficient sensitivity of reagents or virus variation.
SARS-CoV-2 is a single-stranded RNA virus, and its genomes mutate more frequently. The 3-target testing with mutual verification of each target's results can improve the detection rate and help to confirm the diagnosis as early as possible. Fosun Diagnostics' SARS-CoV-2 Nucleic Acid Test Reagent can detect 3 targets (ORF1ab, N and E genes) at the same time. The primer probe sequences used are self-developed and designed, which are different from the segments expanded in the reagents of other manufacturers. This reagent has a sensitivity of up to 300 copies/mL, which can improve the detection rate and effectively reduce missed cases and retests.